ePulse Solution Framework
A modular, digitalization production monitoring system that strengthens compliances
improves quality throughout by providing shop floor transparency and controlled
manufacturing activities.
ePulse addresses the existing operational challenges across the end to end pharma value
chain through technology led continuous
improvement and innovation.
Its different modules not only help to document every step in the production processes to
be compliant with today’s ever more stringent regulatory mandates, but also its IoT
technology component coupled with the use of advanced analytics is revolutionising how
pharma manufacturing function.
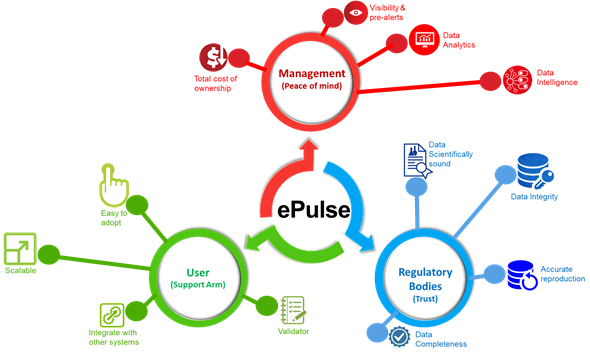
Monitor shopfloor Pulse with ePulse
eLog
Digitizing Log Journey
Presenting Electronic Logbook, complying with 21 CFR Chapter 11
requirements, a simple yet controlled platform for all Pharmaceutical
manufacturing to manage logbooks, digitally.
Complying with GMP guidelines, Electronic Logbook provides capability of
defining flexible workflow, SOPs on areas or equipment and building
validation control points and template builder to design templates.
Intelligence in system alerts in case there is a deviation from laid
procedures,
thus ensures compliance and data integrity.
Being a digital platform, it eliminates use of paper logs and with
available
data from digital logbooks, it further provides analytical capabilities
for
operational efficiency improvements which cannot be achieved using
manual
logbooks.
eOS
Equipment Agnostic IoT
Utilization is a key parameter for calculating OEE, eOS captures
single point of truth without directly integrating with the
equipment. eOS is a nimble IoT component which feeds pulse of
equipment on a real-time basis.
eOS, a non-invasive IoT component which can be attached to any
equipment or a machine to get a pulse of machine running or idle
status.
On
Status
Running
Status
Idle / Off
status
iMMS
Intelligent Manufacturing Monitoring System
iMMS is ERP/machine agnostic system providing capabilities of
real time data acquisition & production monitoring. It
integrates with available SCADA and PLC to acquire machine /
equipment data on a real-time basis.
Acquired data provides real-time / anytime process sheets and
also available as digital backup for future data archival. Data
acquisition further empowers alert & event logs which can be
proactively be used for required actions.
Data analytics provides insights on Batch Performance,
Equipment OEE, Breakdown patterns and manpower
utilization & efficiencies.
Utilization
Data Intelligence
Turning information into insights
ePulse not only converts value to data but also provides enablement
to businesses to improve safety & risk management.
It enables business to increase production of batches, reduction in
downtime and an improved OEE.
Data correlations provides insights on possible capacity release, route optimizations, quality / supervisor performance, manpower utilization and other multi-dimensional analysis.
Advantages
Designed with inputs from best of Pharma process owners & thus providing a Prebuild Module.
Agile Template Builder – Capabilities to build customized templates adopted at work environment
Powerful analytics for improved OEE and data insights
Integration compatibility with SAP / Other ERP Systems, LIMS and eLabs using web services or APIs
Simple UI/UX which makes the solution easy to understand & adopt.
Powerful workflow engine to ensure all tasks are executed as per defined workflow with digital signatures.
Multiple logging options other than login & passwords like Iris or face recognition .
Modular approach, thus provide easy scalability
Complies to GMP guidelines and 21CFR Chapter 11